Once a scientific marvel, CAR-T (Chimeric Antigen Receptor T-cell) therapy is now a billion-dollar frontier reshaping cancer care, investment, and innovation.
What is CAR-T Therapy?
CAR-T is a personalized immunotherapy where a patient’s own T cells are extracted, genetically modified to target cancer cells, and then reinfused. This “living drug” teaches the immune system to attack cancer with precision. It’s a multi-step process—leukapheresis, genetic engineering, and re-infusion—followed by monitoring for side effects like cytokine release syndrome (CRS) and neurotoxicity, which are manageable at specialized centers.
Breakthrough Outcomes
CAR-T therapy has delivered unprecedented results, especially in blood cancers like leukemia, lymphoma, and multiple myeloma. Real-world data shows 80–90% response rates, with many patients achieving long-term remission—and even cures. Individuals previously referred for hospice are now walking out cancer-free.
FDA Milestones and Evolving Guidelines
Since the FDA approved the first CAR-T (Kymriah) in 2017, over six therapies have entered the market, with NCCN and ASCO now recommending CAR-T earlier in treatment for certain cancers. Trials are even testing its use as frontline therapy.
Beyond Blood Cancers
Though current successes lie in hematologic cancers, efforts are underway to tackle solid tumors (lung, breast, brain, pancreas) using localized delivery and multi-targeted CARs. CAR-T is also being explored for autoimmune diseases like lupus and fibrotic conditions, expanding its future reach far beyond oncology.
Why Investors Are Watching
The U.S. CAR-T market is expected to grow from $3B in 2023 to over $10B by 2030, with global projections reaching $61B–$129B by 2034. Growth is driven by rising cancer rates, expanding indications, advanced manufacturing, and massive pharma investments. Biotech firms like BMS, Novartis, J&J, and Legend Biotech are all-in.
Tech Meets Medicine
AI is optimizing CAR-T across the board—from designing safer, more effective CARs to predicting patient response and side effects. Smart manufacturing, including AI-monitored bioreactors and automation, is reducing production time from weeks to days. This convergence of bioengineering and tech is streamlining delivery and expanding access. These advancements were a key focus at the recent Digital Health Innovation Summit, where CAR-T’s future was spotlighted as a leading frontier of tech-driven care.
Policy & Reimbursement
Medicare covers all FDA-approved CAR-T therapies, reimbursing hospitals significantly. Private insurers also cover CAR-T but require treatment at certified centers. Innovative models like pay-for-response and installment-based reimbursement are being explored. Victim assistance programs are helping bridge access gaps for those facing high out-of-pocket costs.
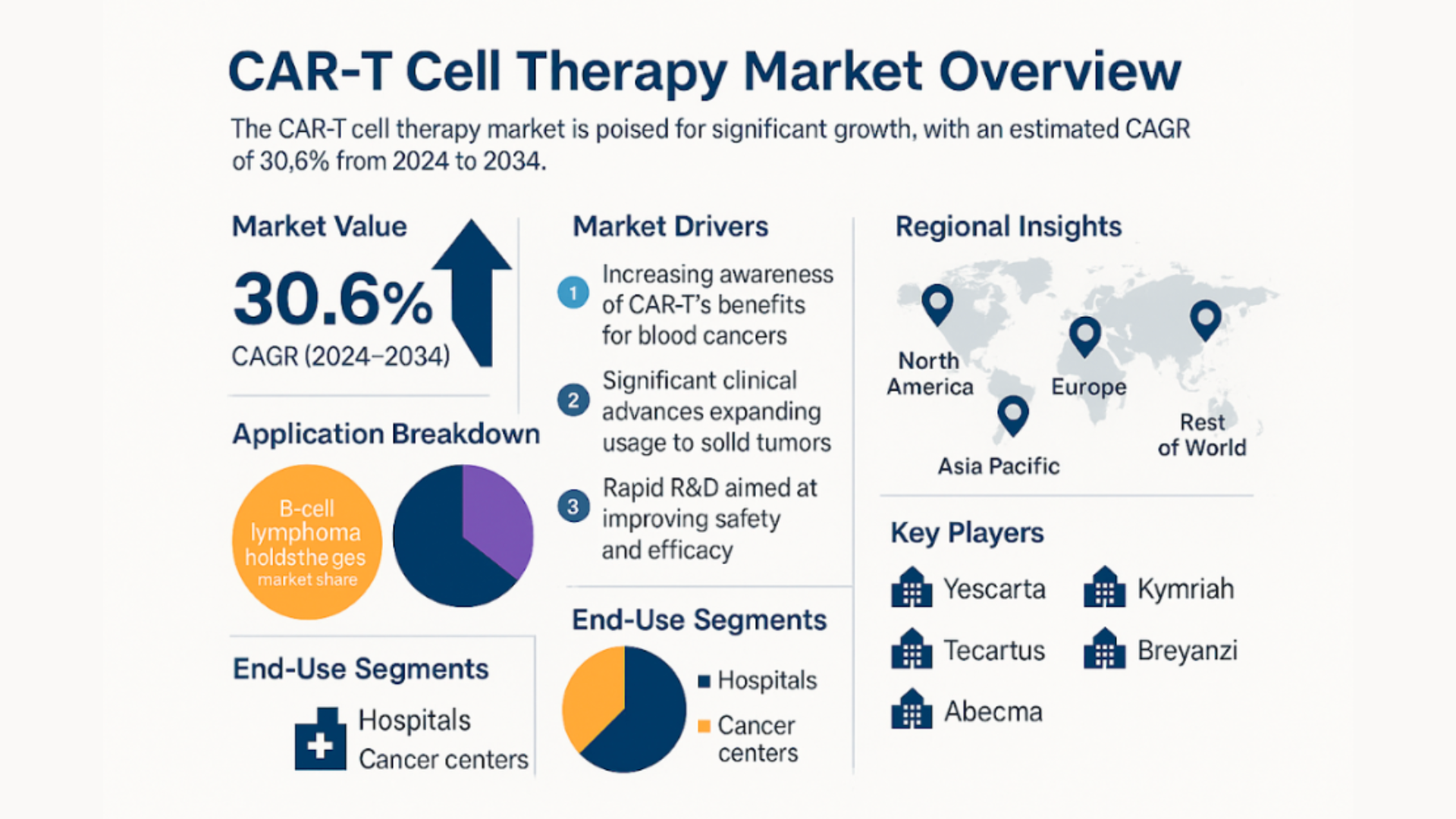
Market Insights
Final Thought
CAR-T therapy represents a paradigm shift in precision medicine—offering renewed hope for patients once deemed untreatable. For clinicians, it’s a revolutionary tool. For patients, it’s a second chance. For investors and innovators, it’s a rapidly maturing space blending science, technology, and high impact. In many ways, we’re not just treating cancer—we’re rewriting biology’s rulebook.





